
Competitive Exams / NEET CHEMISTRY / CHEMISTRY-NEET
Sachin Verma
Masters of Science from Indian Institute of Technology, Kanpur
Student's Reviews
By Several Parameters
NEET Syllabus 2023 includes topics covered in Class 11 and 12 Physics, Biology, and Chemistry syllabus. National Eligibility cum Entrance Test (NEET) is a medical entrance examination conducted nationwide by the NTA (National Testing Agency) for students aspiring to pursue medical courses across medical colleges in the country. NEET 2023 question paper is formed on the basis of the syllabus of NEET 2023 specified by the MCI (Medical Council of India).
1. Must have a 10+2 or equivalent diploma from a recognised board, with Physics, Biology/Biotechnology, Chemistry, and English as core courses.
2. Aspirers with a Class 12 or equivalent are also eligible to apply for NEET.
| Course | Fee per Class (In KlassCoins) | Duration | Type |
|---|---|---|---|
| CHEMISTRY-NEET | 810 | 11 Months | Indiviual Classes |
Hii all this side I am Sachin Verma a Chemistry enthusiast from India, currently as a Faculty of Chemistry at CSRL Super 30, Jammu. I am here to get associated with learning minds so that I can impart all the knowledge which I have gained over the years from various work experience with different institutes and shape the student for future.
My principle subject is Chemistry so I mainly teach students seeking help in Chemistry belonging to all boards. I am concerned with teaching chemistry for IIT-JEE and NEET.
October 2022 - December 2023:
CSRL, Jammu Super 30 - Chemistry Faculty,
I am a Chemistry faculty at CSRL, Jammu Super 30. Where I teach JEE/Advanced level Chemistry. I have four years of teaching experience in both offline and online modes.
-
Subject Details:
My principle subject is Chemistry so I mainly teach students seeking help in Chemistry belonging to all boards. I am concerned with teaching chemistry for IIT-JEE and NEET. Along with this I help student in understanding Science and Maths subject upto Grade -10.
I have experince in the following institute:
- Four Year Teaching Experience of Competition level Exam (Jee Main, Neet & Jee Advanced) (2019- 23)
- Two year Teaching Experience (IIT JAM, IIT Gate, CSIR-Net JRF Exam) (2018-20)
- Chegg Subject Matter Expert (Chemistry) Active From (April-2020)
- Lecturer in Chemistry @ DEI REI Intermediate College, Agra (2020-2021, 2021-2022)
Apart from academics knowlede I also have technical skills like :
- Operating Systems: Windows , Linux
- Other tools: MATLAB, MS-Office, Origin, Chem-Draw, Fortran, Gaussian software, Latex (Basics)
- Laboratory Techniques: Thin-layer chromatography(TLC), Column chromatography, Canola Filtration, NMR-Analysis, Mass-spectroscopy analysis, Infrared spectroscopy (IR), Ultra-violet spectroscopy(UV) , To detect surface images by Atomic Force Microscopy (AFM) , To detect non-linear optical property by Laser technique (MIRA-900 F) and X-ray diffraction (XRD)
-
Book Published Details:
Carcinogenicity of Hexavalent Chromium and Its Effect. (Bentham Publication)
-
Language Known:English, Hindi
-
Total Experiance:4 Years
-
Listening & Questioning Skill:Good
-
Reading Skill:Good
-
Writing Skill:Good
-
Presentation Skill:Good
-
Online classes Experiance:2000 hours
-
Award Recg:
- Secured All India General Rank 360 in IIT JAM Exam (Feb-2015)
- Secured All India Rank 124 in CSIR-JRF (June-2016)
- Secured All India Rank 121 in UGC-JRF (Dec-2016)
- Qualifying GATE-2017, GATE-2019 & Gate-2021 Exam
-
Research Work:
- “Investigating the effects of intermolecular interaction on nonlinear optical (NLO) properties of binary mixtures with high repetition rate(HRR) femtosecond laser”. Rahul Kumar Gupta, Sachin Verma, Sumit Singhal, Surya Kant and Debabrata Goswami.
- “First report on bioaccumulation kinetics of Chromium (VI) and Malachite green by Colchicum luteum from aqueous medium”. Ujjwal Kumar1, Ashok K. Jha2*, Shailesh Kumar2 and Sachin Verma2
- “Studies of Modified bentonite for Removal of Cr(VI) from aqueous medium”. A.K. Jha1, S. Majumder2, Sachin Verma2, P. Kumari2, S. Kumar2 and Usha Sharma2
- “Removal of Cr(VI) from aqueous medium by Cynodon dactylon and orange peel powder”. Kiran Kumari1, Sachin Verma2, A.K. Jha1 and Usha Sharma2
- “Rare Earth and Trace Metal Characteristics of Bentonite in the Rajmahal Hills” A.K. Jha1, Raghbendra Thakur2, Sachin Verma3, Subhajit Sikdar4
- "Geochemical mobilization of Arsenic, Chromium and Uranium in Gangetic Plain of Bihar and Jharkhand" (Accepted)
- Models of adsorption for adsorption of N2 on Rajmahal and Rajasthan bentonite and modified bentonite. (Under Review
Degree: Bachelors of Science - University: Dr. Bhim Rao Ambedkar University, Agra
Degree: Masters of Science - University: Indian Institute of Technology, Kanpur
Degree: B.Ed - University: Dr. Bhimrao Ambedkar University, Agra
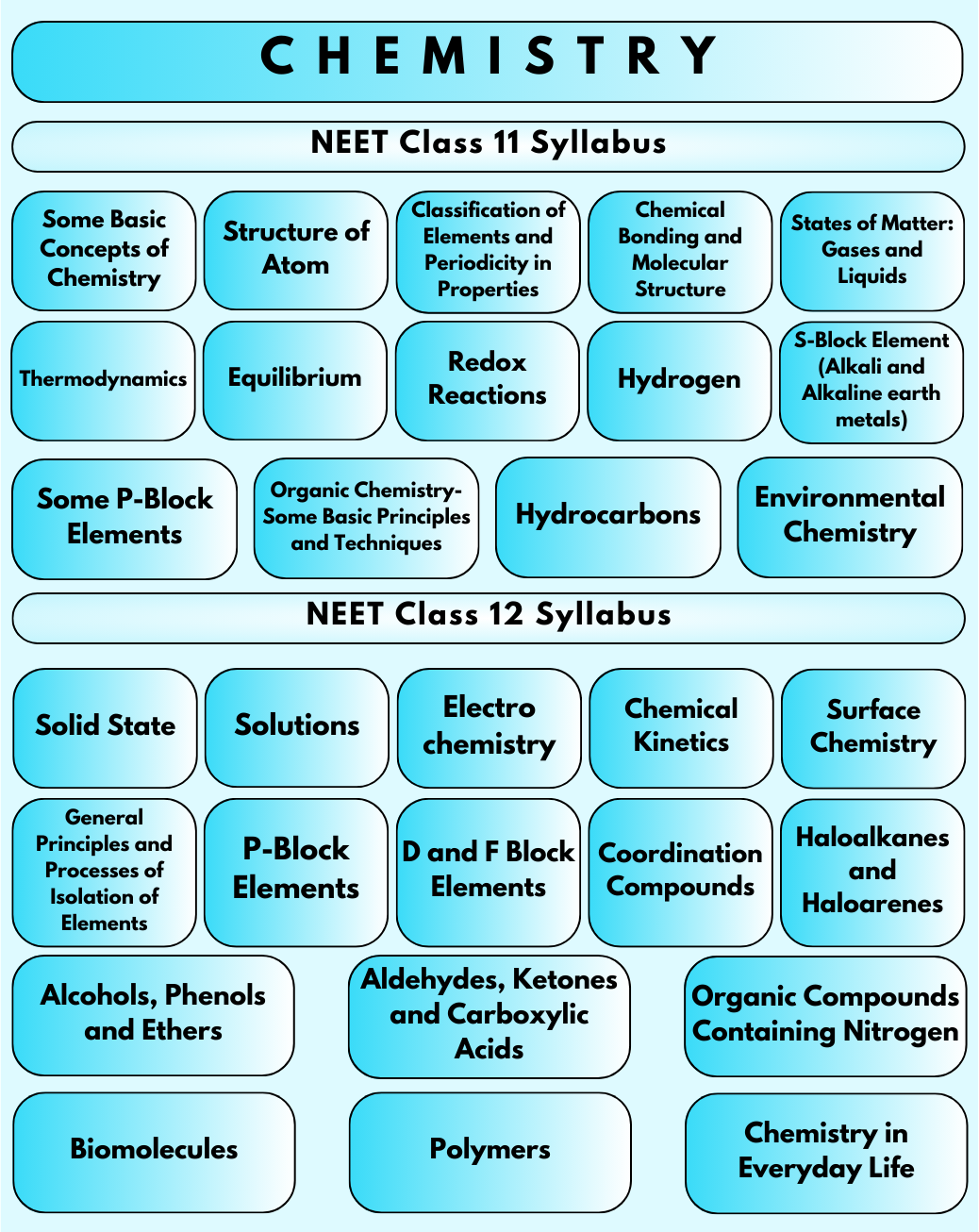
NEET Syllabus 2023 includes topics covered in Class 11 and 12 Physics, Biology, and Chemistry syllabus. National Eligibility cum Entrance Test (NEET) is a medical entrance examination conducted nationwide by the NTA (National Testing Agency) for students aspiring to pursue medical courses across medical colleges in the country. NEET 2023 question paper is formed on the basis of the syllabus of NEET 2023 specified by the MCI (Medical Council of India).

-
Learning Objectives:
The NEET Chemistry syllabus has been revised for the 2024-25 session. The syllabus has been reduced with nearly 9 units being eliminated from the previous syllabus. This change aims to streamline the syllabus and emphasize the fundamental concepts crucial for medical education. Therefore, students are advised to check the latest syllabus to prepare for NEET 2024.
The NEET Chemistry syllabus covers both 11th and 12th grade material. To ensure a comprehensive understanding and avoid any confusion during NEET preparation, students must thoroughly study the prescribed topics. The National Testing Agency (NTA) will conduct the National Eligibility cum Entrance Test (NEET) 2024 solely based on the revised syllabus.
The NEET Chemistry Syllabus consists of physical, organic and inorganic chemistry.
-
Course Outline:
Physical Chemistry Syllabus for NEET
There are a total of eight units under the Physical Chemistry section. The unit-wise chapters and topics are given below.
Unit I: Some Basic Concepts of Chemistry
Matter and its nature, Dalton's atomic theory: Concept of atom, molecule, element. and compound. Laws of chemical combination; Atomic and moleculat masses, mole concept, molar mass, percentage composition, empirical and molecular formulae: Chemical equations and
stoichiometry.Unit II: Atomic Structure
Nature of electromagnetic radiation, photoelectric effect; Spectrum of the hydrogen atom. Bohr model ofa hydrogen atom - its postulates, derivation ofthe relations for the energy ofthe electron and radii of the different orbits, limitations of Bohr's model; Dual nature of r,latter, de Broglie's relationship. Heisenberg uncertainty principle. Elementary ideas ofquantum mechanics, quantum mechanics, the quantum mechanical model of the atom, its important features. Concept of atomic orbitals as one-electron wave functions: Variation of Ψ and Ψ 2 with r for 1s and 2s orbitals: various quantum numbers (principal, angular momentum, arrd magnetic quantum numbers) and their slgnificance; shapes of s, p, and d - orbitals, electron spin and spin quantum number: Rules for filling electrons in orbitis - Aufbau principle. Pauli's exclusion principle and Hund's rule, electronic configuration of elements, extra stability of half-filled and completely filled orbitals.
Unit III: Chemical Bonding and Molecular Structure
Kossel - Lewis approach to chemical bond formation, the concept of ionic and covalent bonds.
Ionic Bonding: Formation of ionic bonds, factors affecting the formation of ionic bonds; calculation of lattice enthalpy.
Covalent Bonding: Concept of electronegativity. Fajan's rule, dipole moment: Valence Shell Electron Pair Repulsion (VSEPR) theory and shapes of simple molecules.
Quantum mechanical approach to covalent bonding: Valence bond theory - its important f'eatures. the concept of hybridization involving s, p, and d orbitals; Resonance.
Molecular orbital Theory - Its important features. LCAOs, types of molecular orbitals (bonding, antibonding), sigma and pi-bonds, molecular orbital electronic configuralions of homonuclear diatomic molecules, the concept of bond order, bond length, and bond energy.
Elementary idea of metallic bonding. Hydrogen bonding and its applications.Unit IV: Chemical Thermodynamics
Fundamentals of thermodynamics: System and surroundings, extensive and intensive properties' state functions, types of processes.
The first law of thermodynamics - Concept of work, heat internal energy and enthalpy, heat capacity, molar heat capacity; Hess's law of constant heat summation; Enthalpies of bond dissociation, combustion' formation, atomization. sublimation. phase transition, hydration. ionization. and solution.
The second raw of thermodynamics - Spontaneity of processes: AS ofthe universe and AC of the system as criteria for spontaneity. Standard Gibbs energy change and equilibrium constant.
The following paragraphs bring the important topics from the NEET syllabus 2024 for Chemistry and their weightage.
Unit V: Solutions
Different methods for expressing the concentration of solution - molality, molarity, mole fraction. percentage (by volume and mass both), the vapour pressure of solutions and Raoult's law - Ideal and. non-ideal solutions, vapour pressure - composition, plots for ideal and non-ideal solutions: colligative properties of dilute solutions - a relative lowering of vapour pressure, depression of freezing point, the elevation of.boiling point and osmotic pressure; Determination of molecular mass using colligative properties; Abnormal value of molar mass, Van't Hoff Factor and its significance.
Unit VI: Equilibrium
Meaning of equilibrium, the concept of dynamic equilibrium.
Equilibria involving physical processes: Solid-iquid, liquid - gas and sorid-gas equiribria, Henry's law. General characreristlcs of equilibrium involving physical processes.
Equiribrium invorving chemica processes: Law of chemical equilibrium, equiribrium constants (Kp and Kc) and their significance, the significance of ΔG and ΔG° in chemical equiribrium, factors affecting equilibrium concentration, pressure, temperature, the effect of catalyst; Le Chatelier's principle.
Ionic equiribrium: weak. and strong electrolytes, ionization of electrolytes, various concepts of acids and bases (Arrhenious and Bronsted - Lowry and Lewis) and their ionization, acid-base equilibria (including multistage ionization) and ionization constants, ionization of water. PH scale, common ion effect, hydrolysis of salts and PH of their solutions, the solubility of sparingly soluble salts and solubility products, buffer solutions.
Unit VII: Redox Reactions and Electrochemistry
Electronic concepts of oxidation reduction, redox reactions, oxidation numbers, rules for assigning oxidation number, balancing of redox reactions.
Electrolytic and metallic conduction, conductance in electrolytic solutions, molar conductivities and their variation with concentration, Kohlrausch's law and its applications.
Electrochemical Cells - Electrolytic and Galvanic cells, different types of electrodes, electrode potentials including standard electrode potential, half - cell and cell reactions, emf of a Galvanic cell and its measurement: Nernst equation and its applications; Relationship between cell potential and Gibbs' energy change: Dry cell and lead accumulator; Fuel cells.
Unit VIII: Chemical Kinetics
Rate of a chemical reaction, factors affecting the rate of reactions: concentration, temperature, pressure,'and catalyst; elementary and compiex reactions,order and morecurariry of reactions, rate law, rate constants and its units, differential and integral forms of zero and first-order reactions, their characteristics and half lives, the effect of temperature on the rate of reactions, Arrhenius theory, activation energy and its calculation, collision theory of bimolecular gaseous reactions (no derivation).
Also Read: Physical Chemistry Chapters for NEET
Inorganic Chemistry Syllabus for NEET
This section has four units.
Unit IX: Classification in Elements and Periodicity in Properties
Modern periodic law and present form of periodic table, s, p, d and f block elements, periodic trends in properties of elements atomic and ionic radii, ionization enthalpy, electron gain enthalpy, valence, oxidation states, and chemical reactivity.
Unit X: P-Block Elements
Group 13 to Group 18 Elements
General Introduction: Electronic configuration and general trends in physical and chemical properties of elements across the periods and down the groups; unique behaviour of the first element in each group.
Unit XI: d and f Block Elements
Transition Elements
General introduction, electronic configuration, occurrence and characteristics, general trends in properties, of the first row transition elements - physical properties, ionization enthalpy, oxidation states, atomic radii, colour, catalytic behaviour, magnetic properties, complex formation, interstitial compounds, alloy formation; Preparation, properties and uses of K2Cr207 and KMn04.
Inner Transition Elements
Lanthanoids - Electronic configuration, oxidation states, and lanthanoid contraction.
Actinoids - Electronic configuration and oxidation states.
Unit XII: Co-ordination Compounds
Introduction to coordination compounds.Wemer's theory; ligands, coordination number. denticity. chelation; IUPAC nomenclature of mononuclear co-ordination compounds, isomerism: Bonding-Valence bond approach and basic ideas of Crystal field theory, colour and magnetic properties; lmportance of co-ordination compounds (in qualitative analysis. extraction of metals and in biological systems).
Also Read: Inorganic Chemistry Chapters for NEET 2024 Preparation
Organic Chemistry Syllabus for NEET
This part has the highest number of units, which is eight. Let us take a look at the Organic Chemistry syllabus for NEET.
Unit XIII: Purification and Characterisation of Organic Compounds
Purification - Crystallization. sublimation, distillation, differential extraction, chromatography - principles and their applications.
Qualitative analysis - Detection of nitrogen, sulphur, phosphorus and halogens.
Quantitative analysis (basic principles only) - Estimation of carbon. hydrogen. nitrogen. halogens. sulphur. phosphorus.
Calculations of empirical formulae and molecular formulae: Numerical problems in organic quantitative analysis.
Unit XIV: Some Basic Principles of Organic Chemistry
Tetravalency of carbon: Shapes of simple molecules - hybridization (s and p): crassification of organic compounds based on functional groups: and those containing halogens, oxygen, nitrogen and sulphur; Homologous series: Isomerism - structural and stereoisomerism.
Nomenclature (Trivial and IUPAC)
Covalent bond fission - Homolytic and heterolytic: free radicals. carbocations. and carbanions: stability of carbocations and free radicals. electiophires. and nucleophyles.
Electronic displacement in a covalent bond
Inductive eflect, electromeric eflect. resonance and hyperconjugation.
Common types of organic reactions- Substitution. addition. elimination, and rearrangement.
Unit XV: Hydrocarbons
Classification' isomerism. IUPAC nomenclature, general methods of preparation, properties, and reactions.
Alkanes - Conformations: Sawhorse and Newman projections (of ethane): Mechanism of halogenation of alkanes, projections (of ethane).
Alkenes - Geometrical isomerism: Mechanism of electrophilic addition: addition of hydrogen. halogens, water. hydrogen halides (Markownikoffs and peroxide effect): Ozonolysis and polymerization.
Alkynes - Acidic character: Addition of hydrogen, halogens, water, and hydrogen halides: Polymerization.
Aromatic hydrocarbons - Nomenclature. benzene - structure and aromaticity: Mechanism of substitution: halogenation, nitration.
Friedel- Craft's alkylation and acylation, directive influence of the functional group in mono-substituted benzene.
Unit XVI: Organic Compounds Containing Halogen
General methods of preparation, properties, and reactions; Nature of C-X bond: Mechanisms of substitution reactions.
Uses; Environmental effects of chloroform, iodoform freons, and DDT.
Unit XVII: Organic Compound Containing Oxygen
General methods of preparation, properties, reactions, and uses.
Alcohols, Phenols and Ethers
Alcohols: Identification of primary, secondary, and tertiary alcohols: mechanism of dehydration.
Phenols: Acidic nature, electrophilic substitution reactions: halogenation. nitration and sulphonation. Reimer - Tiemann reaction.
Ethers: Structure.
Aldehyde and Ketones: Nature of carbonyl group; Nucleophilic addition to >C=O group, relative reactivities of aldehydes and ketones; Important reactions such as - Nucleophilic addition reactions (addition of HCN. NH3 and its derivatives), Grignard reagent; oxidation: reduction (Wolf Kishner and Clemmensen); the acidity of α-hydrogen. aldol condensation, Cannizzaro reaction. Haloform reaction, Chemical tests to distinguish between aldehydes and Ketones.
Carboxylic Acids
Acidic strength and factors affecting it.
Unit XVIII: Organic Compounds Containing Nitrogen
General methods of preparation. Properties, reactions, and uses.
Amines: Nomenclature, classification structure, basic character, and identification of primary, secondary, and tertiary amines and their basic character.
Diazonium Salts: Importance in Synthetic Organic Chemistry.
Unit XIX: Biomolecules
General introduction and importance of biomolecules.
CARBOHYDRATES - classification; aldoses and ketoses: monosaccharides (glucose and fructose) and constituent monosaccharides of oligosaccharides (sucrose, lactose, and maltose)
PROTEINS.Elementary Idea of α-amino acids, peptide bond, polypeptides.Proteins: primary. secondary, tertiary, and quaternary structure (qualitative idea only), denaturation of proteins enzymes.
VITAMINS - Classification and functions
NUCLEIC ACIDS - Chemical constitution of DNA and RNA
Biological functions of nucleic acids
Hormones (General Introduction)
Unit XX: Principles Related to Practical Chemistry
Detection of extra elements (Nitrogen, sulphur, halogens), in organic compounds; Detection of the following functional group, hydroxyl (alcoholic and phenolic), carbonyl (aldehyde and ketones) carboxyl, and amino groups in organic compounds.
-
The chemistry involved in the preparation of the following:
Inorganic compounds: Mohr's salt. potash alum
Organic compounds: Acetanilide. p-nitro acetanilide, aniline yellow, iodoform
-
The chemistry involved in the titrimetric exercises - Acids. bases and the use of indicators. oxalic-acid vs KMnO4. Mohr's salt vs KMnO4
-
Chemical principles involved in the qualitative salt analysis
Cations
Anions
Chemical principles involved in the following experiments:
1. Enthalpy of solution of CuSO4
2. Enthalpy of neutralization of strong acid and strong base
3. Preparation of lyophilic and lyophobic sols
4. Kinetic study of the reaction of iodide ions with hydrogen peroxide at room temperature.
There are some units which have been completely removed from last year such as Polymers, Environmental Chemistry, Chemistry in Everyday Lives, Structural Chemistry, etc. The following table brings the list of topics along with their corresponding units which have been removed by NMC.
-
-
Recomended Audience:
The NEET eligibility criteria is a foundational framework that filters out who can participate in this crucial medical entrance exam. To be eligible, candidates must have completed their Class 12 board examination or be in the process of doing so.
-
Pre-Requisite Requirement:
1. Must have a 10+2 or equivalent diploma from a recognised board, with Physics, Biology/Biotechnology, Chemistry, and English as core courses.
2. Aspirers with a Class 12 or equivalent are also eligible to apply for NEET.
-
Course Level:Intermediate
-
Language of Teaching:English, Hindi
-
Class Schedule Availiability:Morning




